Cellular Energy Sensor Reveals New Pathway for Fighting Infections
Published: 29 May 2025
The immune system’s energy sensor: meet the protein that powers up when danger strikes. Researchers have uncovered how a giant cellular protein senses energy surges to launch defences against infection.
Scientists at the MRC-University of Glasgow Centre for Virus Research, in collaboration with the MRC Protein Phosphorylation and Ubiquitylation Unit in Dundee, and the Institute of Molecular Pathology in Vienna, have uncovered a surprising new mechanism that helps our cells detect and fight off a wide range of dangerous pathogens, from viruses to bacteria and parasites. The discovery centres on a massive cellular protein that acts as an energy sensor, providing fresh insights into how our immune systems coordinates defence against infection.
The research, published in Nature Communications, focuses on RNF213, aptly nicknamed "Mysterin" due to its previously mysterious function. This protein giant ranks among the 20 largest in the human genome and has long puzzled scientists with its ability to defend against an unusually diverse array of pathogens—everything from viruses to complex parasites like Toxoplasma and bacteria such as Salmonella.
A Cellular Alarm System Powered by Energy
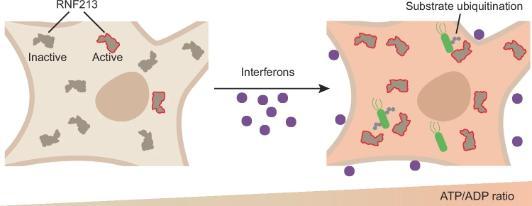
The breakthrough came when researchers discovered that RNF213 functions as a sophisticated energy sensor. When cells are under attack from pathogens, their ATP levels—the universal energy currency used by all living things—naturally rise as part of the immune response. RNF213 detects these elevated ATP levels and springs into action, activating its protective functions.
"This represents a completely new kind of cellular surveillance system," explains the research team. "Rather than looking for specific foreign molecules that mark invaders, RNF213 monitors the cell's own energy state as a sign of infection."
Once activated by ATP, RNF213 functions like a molecular "sticker gun," attaching small protein tags called ubiquitin to other cellular targets. This ubiquitin mark is remarkably protective, helping cells defend against multiple types of pathogens simultaneously.
Bridging Basic Science and Medical Applications
The discovery required an innovative combination of chemical biology, virology, immunology, and structural biology techniques. The researchers developed new methods to track protein activity inside living cells and used advanced imaging to capture the three-dimensional structure of RNF213 in action.
This work reveals the first known example of an ATP-controlled ubiquitin ligase—a finding that could have significant therapeutic implications. Traditional approaches to fighting infections typically target the pathogens themselves, but this research suggests new possibilities for "host-directed therapeutics" that would instead boost the body's own defence mechanisms.
Evolutionary Insights and Future Directions
The findings illuminate how our immune systems have evolved to recognize both foreign invaders and internal danger signals. While cells have long been known to detect pathogen-specific molecules, this research demonstrates how they also monitor their own energy metabolism as an indicator of infection.
"Through millions of years of evolution, our cells have been trained to detect invading microbes," the researchers note. "Now we understand that they're also monitoring their own metabolic state as part of this surveillance system."
Unanswered Questions Drive Future Research
The discovery opens numerous avenues for further investigation. Scientists want to understand why ATP levels rise during infection, how RNF213 distinguishes between normal and danger-related ATP increases, and whether this mechanism can be therapeutically manipulated.
The research also raises intriguing possibilities about other cellular proteins that might function as metabolic sensors during infection; the connection between cellular energy and immune defence may be more widespread than previously recognized.
As antibiotic resistance continues to pose global health challenges, understanding and potentially harnessing these natural defence mechanisms could provide valuable new strategies for treating infectious diseases. The identification of RNF213 as an ATP-responsive immune sensor represents a significant step toward developing next-generation therapeutics that work with, rather than against, our body's evolved defence systems.
This work was only made possible by funding from a UKRI Future Leaders Fellowship, the MRC and BBSRC, the Wellcome Trust, the European Union’s Horizon 2020 research and innovation programme, the Austrian Science Fund and the Vienna Science and Technology Fund.
First published: 29 May 2025
<< News

