Mucus Ninjas - Mihnea Turcanu
Published: 26 January 2018
Mucus is highly underestimated. It's not just snot.
Mucus protects epithelial cells in the visual, auditory, respiratory, gastro-intestinal and urogenital systems. The type of mucus found in the throat is called phlegm, while the one in the lungs is called sputum. In the intestine, mucus lines 2,000 square feet of cells and is produced by goblet cells. It is mainly composed of water and mucin proteins (MUC). In the rat intestine, there are two layers of mucus: a loosely adherent one, which can be scraped off, and a firmly adherent one, which cannot be easily removed (Fig. 1).
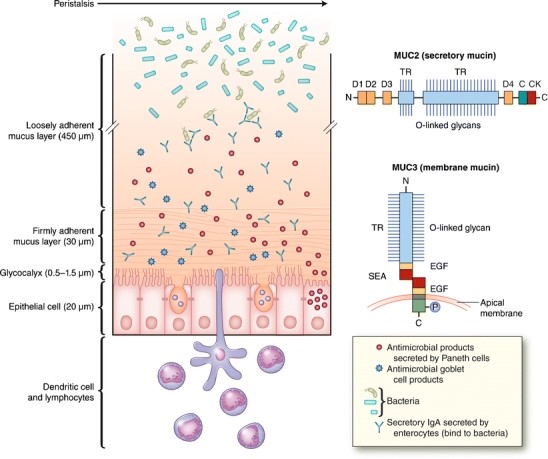
Figure 1. Mucus consists of a loosely and a firmly adherent layer. The firmly adherent layer attaches to the glycocalyx and epithelial cells (Kim et al., 2012).
Mucus has a protective role: it impedes pathogen interaction with the epithelium, it lubricates the intestine during peristalsis – helps transport chyme from the gut to the colon, and protects the cell lining against the acidity in the lumen. The small intestine, except for the distal ileum, presents low microbial content. This is because it flushes chloride (through the CFTR channel) and moves the loose mucus and its bound bacteria distally into the colon. Moreover, the intestinal mucus contains high levels of antibacterial peptides and proteins secreted by both Paneth cells and enterocytes.
It is worth studying mucus because any device or drug in the gut, the reproductive tract or the eye will come into contact with mucus. Performance will be defined by this interaction. Mucus is home to the microbiome and regulates its interactions with the host. Mucus also predicts the fate of pathogens and of drugs which pass through this barrier. Consequently, understanding it can give an insight into predicting and treating infectious diseases. Novel studies research mucus as an alternative to antibiotics. Mucins are linear, elongated, rod-like polymers. Their C-terminals form dimers via disulphide (S-S) bridges, while their N-terminals form trimers via S-S linkages. Mucus is therefore a network cross-linked covalently via disulphide bridges (Fig.2). Mucoadhesivity is due to the many different interactions present in mucus. Besides covalent, disulphide, bonds, there are also electrostatic (van der Walls), hydrophobic and H-bonding interactions. Mucin proteins are rich in oligosaccharides, or sugars, which are suggested to be responsible for the water holding capacity of mucus and the resistance to proteolysis, which might help in maintaining the mucosal barrier.
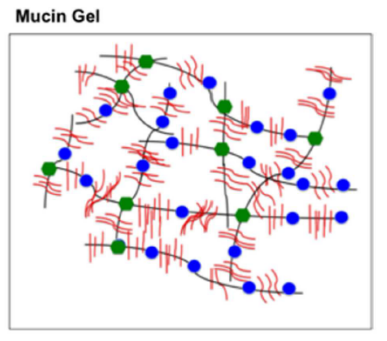
Figure 2. Mucus is a cross-linked network of mucin proteins bound mainly through disulphide bridges (green dots) (Bansil et al., 2013).
Mucus is a non-Newtonian fluid, much like quicksand. Its viscoelastic behaviour depends on pH, ionic strength and degree of hydration. Mucus bounces like a ball when dropped quickly and flows when stretched slowly.
Under high pH values, polymers such as mucus release protons to become negatively charged. Because like-charged parts of the polymer repel each other, the polymer swells. Conversely, under low pH values, there is a solution to gel transition when the mucin solution is sufficiently concentrated. This is due to the interplay between electrostatic and hydrophobic interactions. As the pH is reduced, it flows slower, and does not flow at all at pH 2, because a gel forms at this pH.
The mucus barrier works at three different levels. Firstly, it is a dynamic barrier, where mucus is continuously secreted and shed. Secondly, it is a steric barrier, acting as a molecular sieve with a molecular mass cut off within the range of about 600 to 700 Da. Absorption of substances with higher molecular mass remains at a low level. Thirdly, it is an interactive barrier, where hydrophobic interactions can form between the core of mucin protein and the diffusing compounds (drugs, toxins, other molecules).
Mucus permeability differs for different mucus types. In cystic fibrosis, phlegm was found to hinder foreign molecule mobility with increased particle size. Conversely, in cervical mucus, smaller compounds were immobilized more efficiently.
To sum up, when predicting mucus permeability, one needs to take into account compound size, net charge, lipophilicity and the spatial distribution of charged and hydrophobic groups, besides mucus type and mucus shedding.
Mucins block bacteria adherence, which is necessary for them to clump together and induce harm. When conditioned to remain motile, bacteria can do less harm, suggesting mucus can help the fight against antibiotic resistance. H. Pylori has an interesting mechanism of penetrating the mucus layer to infect the host. The role of the HCl in the stomach is to lower the pH of mucus, inducing it to become a gel which immobilises foreign bodies. H. Pylori secretes urea, which converts the local urea into ammonia and carbon dioxide. Ammonia neutralises HCl, leading to an increase in pH and hence a mucus transition from gel to solution. With the elasticity decreased, H Pylori can swim through the mucus and attach itself to the epithelial wall (Fig.3).
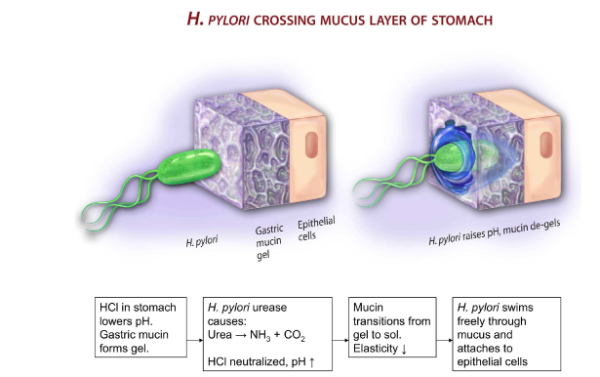
Figure 3. H. Pylori crossing the mucus barrier (Basil et al., 2013).
Mucus is highly underestimated. It is intricate enough that we cannot fully understand it, it represents an effective barrier against pathogens and it serves as a potential alternative to antibiotics. To counteract the general view of mucus being only snot, there are now children stories about superheroes who harvest power from mucus or shoot radioactive mucus through the mouth and nose. Exciting stuff, isn’t it?

First published: 26 January 2018
<< Blog

