Determining processes of CO2 capture with steel slags: implications for mitigating rising CO2 levels
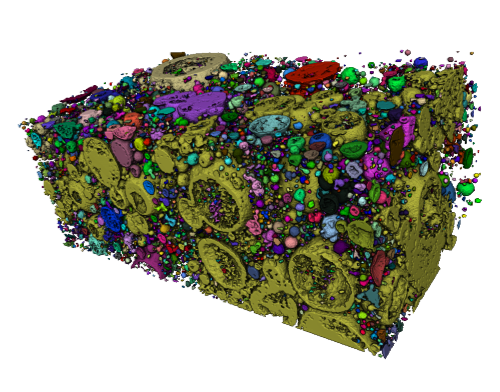
Visualisation of calcite (captured atmospheric CO2) in pores in steel slag, from µCT analysis; long axis is 2mm
(copyright - Dr John MacDonald)
Keywords – CO2, Climate Change Mitigation, Carbon Capture and Storage, Steel Slag
Project Summary: Limiting the CO2 concentration in Earth’s atmosphere, and therefore global temperature rise, is imperative. Conceptually, by-products from certain industrial processes, e.g. steel slag, can passively capture atmospheric CO2 and convert it to solid calcite, thus reducing atmospheric CO2 levels. This project will determine the underpinning science behind the mechanisms of the CO2 capture and precipitation on industrial residues. The insight into these mechanisms will allow for optimisation of the CO2 capture with industrial by-products, so CO2 capture can be maximised in future deployment of this technology.
Observation of heaps of steel slag from former steelworks which have been exposed to the atmosphere for decades shows clear carbonation of the slag. Conceptually, the breakdown of unstable calcium silicate minerals in steel slag results in the formation of new more stable products – different silicate minerals and CaCO3 – calcite. However, pilot analysis by the lead supervisor and collaborators suggests that CO2 capture by steel slag is not a simple single-step precipitation of calcite. A µCT scan of a small fragment of a piece of decades-old steel slag has illustrated calcite lining pores in the slag, with dendritic growth of calcite in the larger pores. There is also an apparent relationship between pore size and whether the pore is filled with calcite. This pilot analysis indicates a complex process of CO2 capture.
Enhanced atmospheric CO2 capture with steel slag has the potential to be transformative in efforts to limit atmospheric CO2 concentrations. Given there are thousands of these legacy slag heaps worldwide, and 250 million tonnes of new slag being generated every year, enhanced CO2 capture with steel slags is a potentially transformative approach to mitigating anthropogenic climate change. However, in order to maximise the amount of CO2 that can be captured with slags, the underpinning science behind the CO2 sequestration process must be known.
This project will address this knowledge gap by utilising a range of microanalytical techniques – focusing on µCT and EBSD (Electron Backscatter Diffraction) to image precipitated calcite in/on steel slag samples. A combination of imaging and chemical analyses will enable the student to shed light on the CO2 capture process.
Project Team: The student will be based in the School of Geographical and Earth Sciences with lead supervisor John MacDonald. Dr MacDonald’s research interests include carbonate chemistry and properties, including that formed through capture by steel slag and other industrial wastes. The supervisory team includes: Dr Neil Burnside (School of Engineering) who has expertise in mineral carbon capture; Dr Luke Daly (School of Geographical and Earth Sciences) who is an expert in EBSD; and Dr Alice Macente (School of Geographical and Earth Sciences) who is an expert in µCT.
The successful candidate will join the small but varied and dynamic PhD cohort of the School of Geographical and Earth Sciences. Due to the interdisciplinary nature of the project, the student will also be affiliated to Dr Burnside’s group in the School of Engineering, and Dr Macente’s µCT collaborators at the University of Strathclyde, thus providing a strong and diverse network for the student.

